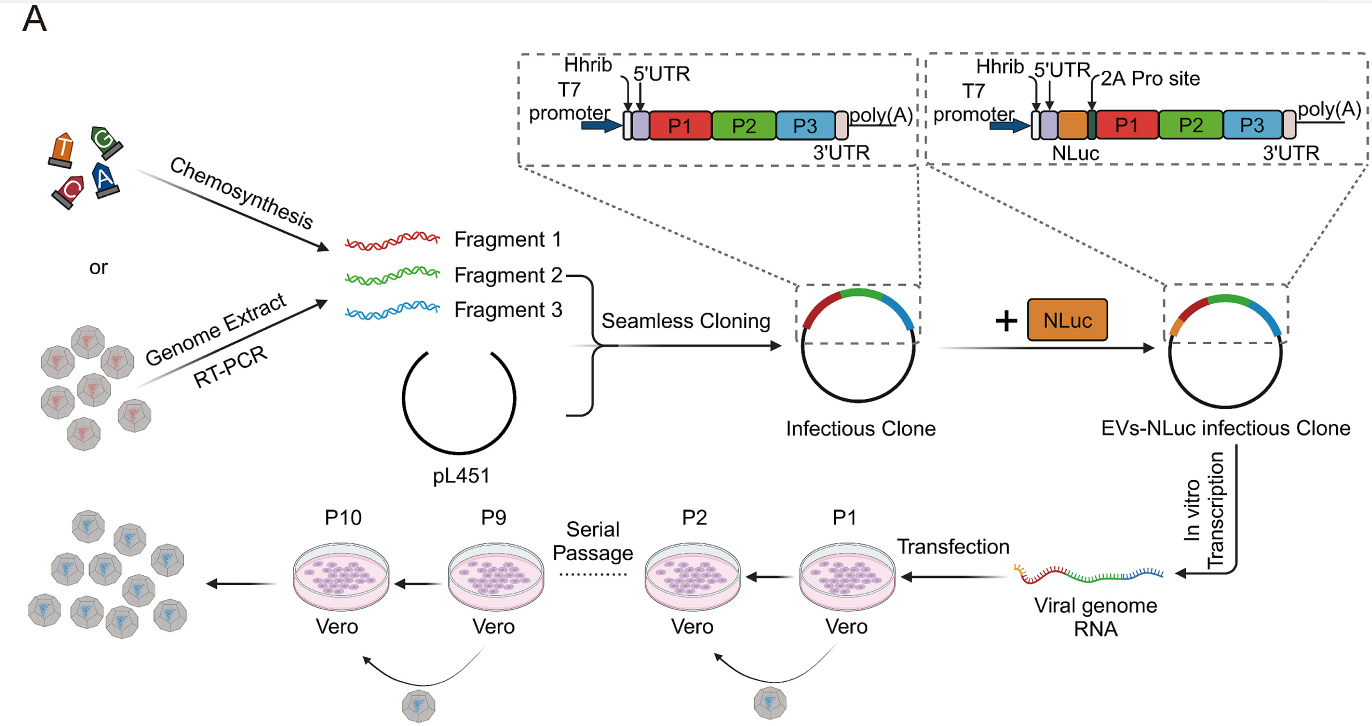
-
Arnold, G.F., Resnick, D.A., Smith, A.D., Geisler, S.C., Holmes, A.K.,Arnold, E., 1996. Chimeric rhinoviruses as tools for vaccine development and characterization of protein epitopes. Intervirology, 39, 72-78.
-
Cao, J.M., Qu, M., Liu, H.T., Wan, X., Li, F., Hou, A., Zhou, Y., Sun, B., Cai, L.J., Su, W.H.,Jiang, C.L., 2020. Myristoylation of EV71 VP4 is essential for infectivity and interaction with membrane structure. Virol. Sin., 35, 599-613.
-
Chen, Y.K., Li, X.H., Wang, M., Li, Y., Fan, J., Yan, J.J., Zhang, S.Y., Lu, L.,Zou, P., 2023. A cysteine protease inhibitor GC376 displays potent antiviral activity against coxsackievirus infection. Curr. Res. Microb. Sci., 5, 100203.
-
Chiu, W.Y., Lo, Y.H.,Yeh, T.C., 2016. Coxsackievirus associated hand, foot and mouth disease in an adult. QJM-an International Journal of Medicine, 109, 823-824.
-
Chou, A.H., Liu, C.C., Chang, J.Y., Jiang, R.N., Hsieh, Y.C., Tsao, A., Wu, C.L., Huang, J.L., Fung, C.P., Hsieh, S.M., Wang, Y.F., Wang, J.R., Hu, M.H., Chiang, J.R., Su, I.J.,Chong, P.C.S., 2013. Formalin-inactivated EV71 vaccine candidate induced cross-neutralizing antibody against subgenotypes B1, B4, B5 and C4A in adult volunteers. PLoS One, 8: e79783.
-
Chu, J.Q., Lee, Y.J., Park, J.N., Kim, S.M., Lee, K.N., Ko, Y.J., Lee, H.S., Cho, I.S., Kim, B.,Park, J.H., 2013. Construction of a bovine enterovirus-based vector expressing a foot-and-mouth disease virus epitope. J. Virol Methods, 189, 101-104.
-
Chua, K.B., Ng, Q., Meng, T.,Jia, Q., 2022. Development of stable, cold-adapted, temperature-sensitive/conditional lethal chimeric enterovirus A71 and coxsackievirus A16. Virol. Sin., 37, 769-773.
-
Deng, C.L., Li, X.D., Liu, S.Q., Xu, L.L., Ye, H.Q., Qin, C.F.,Zhang, B., 2015. Development and characterization of a clinical strain of Coxsackievirus A16 and an eGFP infectious clone. Virol. Sin., 30, 269-276.
-
Gao, Q.Q., Yuan, S.L., Zhang, C., Wang, Y., Wang, Y.Z., He, G.M., Zhang, S.Y., Altmeyer, R.,Zou, G., 2015. Discovery of itraconazole with broad-spectrum, antienterovirus activity that targets nonstructural protein 3A. Antimicrob. Agents Chemother., 59, 2654-2665.
-
Gaspar, N., Zambito, G., Dautzenberg, I.J.C., Cramer, S.J., Hoeben, R.C., Lowik, C., Walker, J.R., Kirkland, T.A., Smith, T.P., Van Weerden, W.M., De Vrij, J.,Mezzanotte, L., 2020. NanoBiT system and hydrofurimazine for optimized detection of viral infection in mice-a novel in vivo imaging platform. Int. J. Mol. Sci., 21, 5863.
-
Hashem, S.M., Gad, M.K., Anwar, H.M., Saleh, N.M., Shamma, R.N.,Elsherif, N.I., 2023. Itraconazole-loaded ufasomes: evaluation, characterization, and anti-fungal activity against. Pharmaceutics, 15, 26.
-
Jubelt, B.,Lipton, H.L., 2014. Enterovirus/picornavirus infections. Handb. Clin. Neurol., 123, 379-416.
-
Kim, C., Kang, H., Kim, D.E., Song, J.H., Choi, M., Kang, M., Lee, K., Kim, H.S., Shin, J.S., Jeong, H., Jung, S., Han, S.B., Kim, J.H., Ko, H.J., Lee, C.K., Kim, M.,Cho, S., 2016. Antiviral activity of micafungin against enterovirus 71. Virol. J., 13, 99.
-
Kimmis, B.D., Downing, C.,Tyring, S., 2018. Hand-foot-and-mouth disease caused by coxsackievirus A6 on the rise. Cutis, 102, 353-356.
-
Liang, X.Y., Zhu, Q.C., Liang, J.Q., Liu, S.Y., Liu, D.X.,Fung, T.S., 2020. Development of HiBiT-tagged recombinant infectious bronchitis coronavirus for efficient and viral quantification. Front. Microbiol., 11, 2100.
-
Lyu, K., Wang, G.C., He, Y.L., Han, J.F., Ye, Q., Qin, C.F.,Chen, R., 2015. Crystal structures of enterovirus 71 (EV71) recombinant virus particles provide insights into vaccine design. J. Biol. Chem., 290, 3198-3208.
-
Miyakawa, K., Jeremiah, S.S., Ohtake, N., Matsunaga, S., Yamaoka, Y., Nishi, M., Morita, T., Saji, R., Nishii, M., Kimura, H., Hasegawa, H., Takeuchi, I.,Ryo, A., 2020. Rapid quantitative screening assay for SARS-CoV-2 neutralizing antibodies using HiBiT-tagged virus-like particles. J. Mol. Cell Biol., 12, 987-990.
-
Nagashima, S., Primadharsini, P.P., Nishiyama, T., Takahashi, M., Murata, K.,Okamoto, H., 2023. Development of a HiBiT-tagged reporter hepatitis E virus and its utility as an antiviral drug screening platform. J. Virol., 97, e0050823.
-
Ren, P.J., Zheng, Y.M., Wang, W.Q., Hong, L.P., Delpeyroux, F., Arenzana-Seisdedos, F.,Altmeyer, R., 2017. Suramin interacts with the positively charged region surrounding the 5-fold axis of the EV-A71 capsid and inhibits multiple enterovirus A. Sci. Rep., 7, 42902.
-
Ren, P.J., Zou, G., Bailly, B., Xu, S.S., Zeng, M., Chen, X.S., Shen, L., Zhang, Y., Guillon, P., Arenzana-Seisdedos, F., Buchy, P., Li, J., Von Itzstein, M., Li, Q.H.,Altmeyer, R., 2014. The approved pediatric drug suramin identified as a clinical candidate for the treatment of EV71 infection-suramin inhibits EV71 infection in vitro and in vivo. Emerg. Microbes Infect., 3: e62.
-
Rezaei, R., Surendran, A., Singaravelu, R., Jamieson, T.R., Taklifi, P., Poutou, J., Azad, T.,Ilkow, C.S., 2021. Detection of SARS-CoV-2 receptor-binding domain antibody using a HiBiT-based bioreporter. J. Vis. Exp., 174: e62488, https://doi.org/10.3791/62488.
-
Sarma, N., 2013. Hand, foot, and mouth disease: current scenario and Indian perspective. Indian J. Dermatol. Venereol. Leprol., 79, 165-175.
-
Shang, B.D., Deng, C.L., Ye, H.G., Xu, W.B., Yuan, Z.M., Shi, P.Y.,Zhang, B., 2013. Development and characterization of a stable eGFP enterovirus 71 for antiviral screening. Antivir. Res., 97, 198-205.
-
Shribman, A.J.,Hanning, C.D., 1986. Hyperbaric bupivacaine and hyperbaric cinchocaine: a comparison of their use for spinal anaesthesia. Eur. J. Anaesthesiol., 3, 103-110.
-
Simmonds, P., Gorbalenya, A.E., Harvala, H., Hovi, T., Knowles, N.J., Lindberg, A.M., Oberste, M.S., Palmenberg, A.C., Reuter, G., Skern, T., Tapparel, C., Wolthers, K.C., Woo, P.C.Y.,Zell, R., 2020. Recommendations for the nomenclature of enteroviruses and rhinoviruses. Arch. Virol., 165, 793-797.
-
Sinclair, W.,Omar, M., 2023. Enterovirus. StatPearls. Treasure Island (FL).
-
Strating, J.R.P.M., Van Der Linden, L., Albulescu, L., Bigay, J., Arita, M., Delang, L., Leyssen, P., Van Der Schaar, H.M., Lanke, K.H.W., Thibaut, H.J., Ulferts, R., Drin, G., Schlinck, N., Wubbolts, R.W., Sever, N., Head, S.A., Liu, J.O., Beachy, P.A., De Matteis, M.A., Shair, M.D., Olkkonen, V.M., Neyts, J.,Van Kuppeveld, F.J.M., 2015. Itraconazole inhibits enterovirus replication by targeting the oxysterol-binding protein. Cell Rep., 10, 600-615.
-
Sumiyadorj, A., Murai, K., Shimakami, T., Kukroki, K., Nishikawa, T., Kakuya, M., Yamada, A., Wang, Y., Ishida, A., Shirasaki, T., Kawase, S., Li, Y.Y., Okada, H., Nio, K., Kawaguchi, K., Yamashita, T., Sakai, Y., Duger, D., Mizukoshi, E., Honda, M.,Kaneko, S., 2022. A single hepatitis B virus genome with a reporter allows the entire viral life cycle to be monitored in primary human hepatocytes. Hepatol. Commun., 6, 2441-2454.
-
Ulferts, R., De Boer, S.M., Van Der Linden, L., Bauer, L., Lyoo, H.R., Mate, M.J., Lichiere, J., Canard, B., Lelieveld, D., Omta, W., Egan, D., Coutard, B.,Van Kuppeveld, F.J.M., 2016. Screening of a library of FDA-approved drugs identifies several enterovirus replication inhibitors that target viral protein 2C. Antimicrob. Agents Chemother., 60, 2627-2638.
-
Van Der Schaar, H.M., Melia, C.E., Van Bruggen, J.a.C., Strating, J.R.P.M., Van Geenen, M.E.D., Koster, A.J., Barcena, M.,Van Kuppeveld, M., 2016. Illuminating the sites of enterovirus replication in living cells by using a split-GFP-tagged viral protein. mSphere, 1, e00104-e00116.
-
Wang, M., Yan, J., Zhu, L., Wang, M., Liu, L., Yu, R., Chen, M., Xun, J., Zhang, Y., Yi, Z.,Zhang, S., 2020. The establishment of infectious clone and single round infectious particles for coxsackievirus A10. Virol. Sin., 35, 426-435.
-
Xu, L.L., Shan, C., Deng, C.L., Li, X.D., Shang, B.D., Ye, H.Q., Liu, S.Q., Yuan, Z.M., Wang, Q.Y., Shi, P.Y.,Zhang, B., 2015. Development of a stable luciferase enterovirus 71 reporter virus. J. Virol. Methods, 219, 62-66.
-
Yu, R., Wang, M., Liu, L., Yan, J., Fan, J., Li, X., Kang, M., Xu, J., Zhang, X.,Zhang, S., 2022. The development and characterization of a stable Coxsackievirus A16 infectious clone with Nanoluc reporter gene. Front. Microbiol., 13, 1101850.
-
Yun, S.I., Song, B.H., Woolley, M.E., Frank, J.C., Julander, J.G.,Lee, Y.M., 2020. Development, characterization, and application of two reporter-expressing recombinant Zika viruses. Viruses-Basel, 12, 572.
-
Zhang, C., Zhang, G., Zhang, Y., Lin, X., Zhao, X., Cui, Q., Rong, L.,Du, R., 2023. Development of an HiBiT-tagged reporter H3N2 influenza A virus and its utility as an antiviral screening platform. J. Med. Virol., 95, e28345.
-
Zhou, Y., Li, J.X., Jin, P.F., Wang, Y.X.,Zhu, F.C., 2016. Enterovirus 71: a whole virion inactivated enterovirus 71 vaccine. Expert Rev. Vaccines, 15, 803-813.
-
Zhu, F.C., Meng, F.Y., Li, J.X., Li, X.L., Mao, Q.Y., Tao, H., Zhang, Y.T., Yao, X., Chu, K., Chen, Q.H., Hu, Y.M., Wu, X., Liu, P., Zhu, L.Y., Gao, F., Jin, H., Chen, Y.J., Dong, Y.Y., Liang, Y.C., Shi, N.M., Ge, H.M., Liu, L., Chen, S.G., Ai, X., Zhang, Z.Y., Ji, Y.G., Luo, F.J., Chen, X.Q., Zhang, Y., Zhu, L.W., Liang, Z.L.,Shen, X.L., 2013. Efficacy, safety, and immunology of an inactivated alum-adjuvant enterovirus 71 vaccine in children in China: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet, 381, 2024-2032.













 DownLoad:
DownLoad: